1. Workflow Is a Biosafety Control
In high-containment laboratories, safety is not only maintained by walls, filters, or PPE—it also depends on how people, samples, and materials move through the space.
Effective biosafety labs are designed around unidirectional workflow, where clean and contaminated zones are clearly separated, and every process supports containment.
2. What Is Unidirectional Workflow?
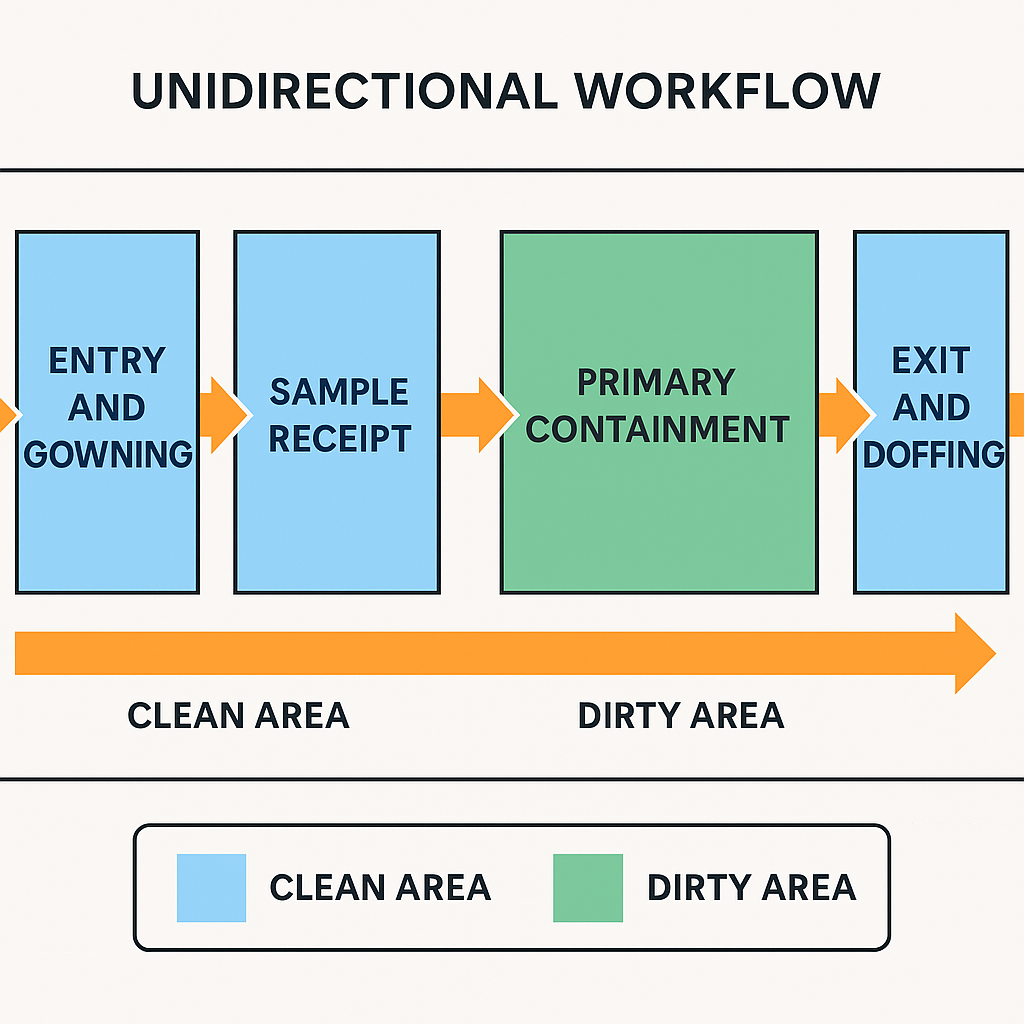
Unidirectional workflow ensures that:
-
Personnel enter and exit through controlled, separate areas
-
Clean zones (e.g., gowning, pre-analytical setup) are upstream of dirty zones (e.g. decontamination, sample processing)
-
Materials and equipment move through dedicated paths
-
Cross-contamination is prevented through physical and procedural separation
Failure to plan this flow early can result in inefficient operation, contamination risks, or the need for costly post-construction modifications.
3. Key Workflow Zones in a biosafety laboratory (BSL)
A well-designed BSL-3 or BSL-2 laboratory should include:
-
Entry and gowning zone: PPE donning, storage, restricted access
-
Sample receipt and preparation area: Pre-analytical separation and biosafety cabinet access
-
Primary containment zone: For culture, extraction, or manipulation under Class II biosafety cabinets
-
Waste and decontamination area: Autoclaves or chemical treatment
-
Exit and doffing area: Separate from entry, with clear removal protocols
Each zone must be supported by the correct airflow, pressure differentials, and material handling systems.

Primary containment equipment such as biological safety cabinets and biological containment centrifuges are at the core of work flow considerations.
4. Workflow Design Affects:
-
Personnel safety: By minimizing exposure to aerosol-generating processes
-
Regulatory compliance: Clear separation of clean and contaminated processes
-
Efficiency: Less downtime, clearer responsibilities, faster response to incidents
-
Maintenance and serviceability: Easier access for equipment servicing without disruption
5. LIS-Labs Builds Containment Around Workflow
At LIS-Labs, workflow isn’t a layer added after construction—it’s a defining part of the lab design process.
We work with technical teams to map sample handling, diagnostic processes, or research protocols and integrate these into the physical layout from the outset.
Our facilities feature:
-
Workflow-specific zoning for sample prep, analysis, and waste control
-
Optimized equipment positioning (biosafety cabinets, pass-throughs, autoclaves)
-
Custom layouts for diagnostics, research, or high-volume testing

